Yahoo Answers is shutting down on May 4th, 2021 (Eastern Time) and beginning April 20th, 2021 (Eastern Time) the Yahoo Answers website will be in read-only mode. There will be no changes to other Yahoo properties or services, or your Yahoo account. You can find more information about the Yahoo Answers shutdown and how to download your data on this help page.
Trending News
Why does [Os2Cl8]2- not form a quadruple bond?
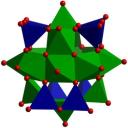
For quadrule bond formation 4 electrons are requried from each metal. Here 5 are available. Does the fact that a pair of e-'s would go into the delta star orbital mean that it is energietically favorable to do something else? The delta star isnt THAT high though... although there is chlorine cholrine eclipising.
Any ideas?
1 Answer
- TimothyLv 68 years agoFavorite Answer
Well, like you've said, you have 5 electrons contributed per Os and that means that if you had an eclipsed Cl₄Os–OsCl₄ geomtery, δ* would be filled. In other words, you'd have a σ²π⁴δ²δ*² configuration. Bond order = (8 – 2)/2 = 3. The two antibonding electrons cancel the δ bond and the bond order goes down to a triple bond. The geometry is consequently staggered, not eclipsed. In the eclipsed geometry, the d-d δ overlap is eliminated; instead, each of the dδ orbitals (on each square-pyramidal OsCl₄ fragment) only overlaps with the highly destabilized d orbital on the other fragment that is pushed up by interaction with the chloride ligand donor orbitals. Basically then, in the staggered geometry, those two δ-symmetry d orbitals remain non-bonding.
EDIT: Apparently the Os₂X₈²⁻ ions USUALLY adopt staggered conformations, but not always (see the 2nd link below). No matter, it should still have only a triple bond, as discussed above.
Source(s): Pictures of the orbitals for a δ-bonded molecule are shown on slide 55 of this pdf file: http://www.chem.tamu.edu/rgroup/hughbanks/courses/... Structure of Os₂Cl₈²⁻ is eclipsed after all: http://books.google.com/books?id=qbNa5eNUIEIC&pg=P...